
Selangor, Malaysia, 8 August 2025 – Reszon Diagnostics International Sdn. Bhd. (“Reszon”), a leading provider of rapid diagnostic solutions, is pleased to announce that the LabPad® Evolution Point of Care (PoC) testing device and its compatible Ksmart® and Tsmart® consumables, manufactured by BioSynex, have successfully obtained approval from the Ministry of Health of the Republic of Indonesia. The certification paves the way for the official market release of the product range across Indonesia.
To accelerate market penetration, Reszon is partnering with PT Pasifik Saintifindo, a well-established medical device distributor in Indonesia, to bring the LabPad® Evolution series to a broad range of healthcare facilities. This includes hospitals, Puskesmas (community health centers), private GP clinics and diagnostic outlets throughout the country.
The LabPad® Evolution is an ultra-portable testing device intended to measure multi-parameters designed for Point-of-Care Testing (POCT) by healthcare professionals. It delivers rapid and reliable quantitative results when paired with single-use compatible consumables, enabling healthcare providers to perform on-the-spot testing without the need for centralized laboratory facilities. This is crucial to support effective and immediate clinical decision-marking especially dealing with critical and emergency cases that every minute count.
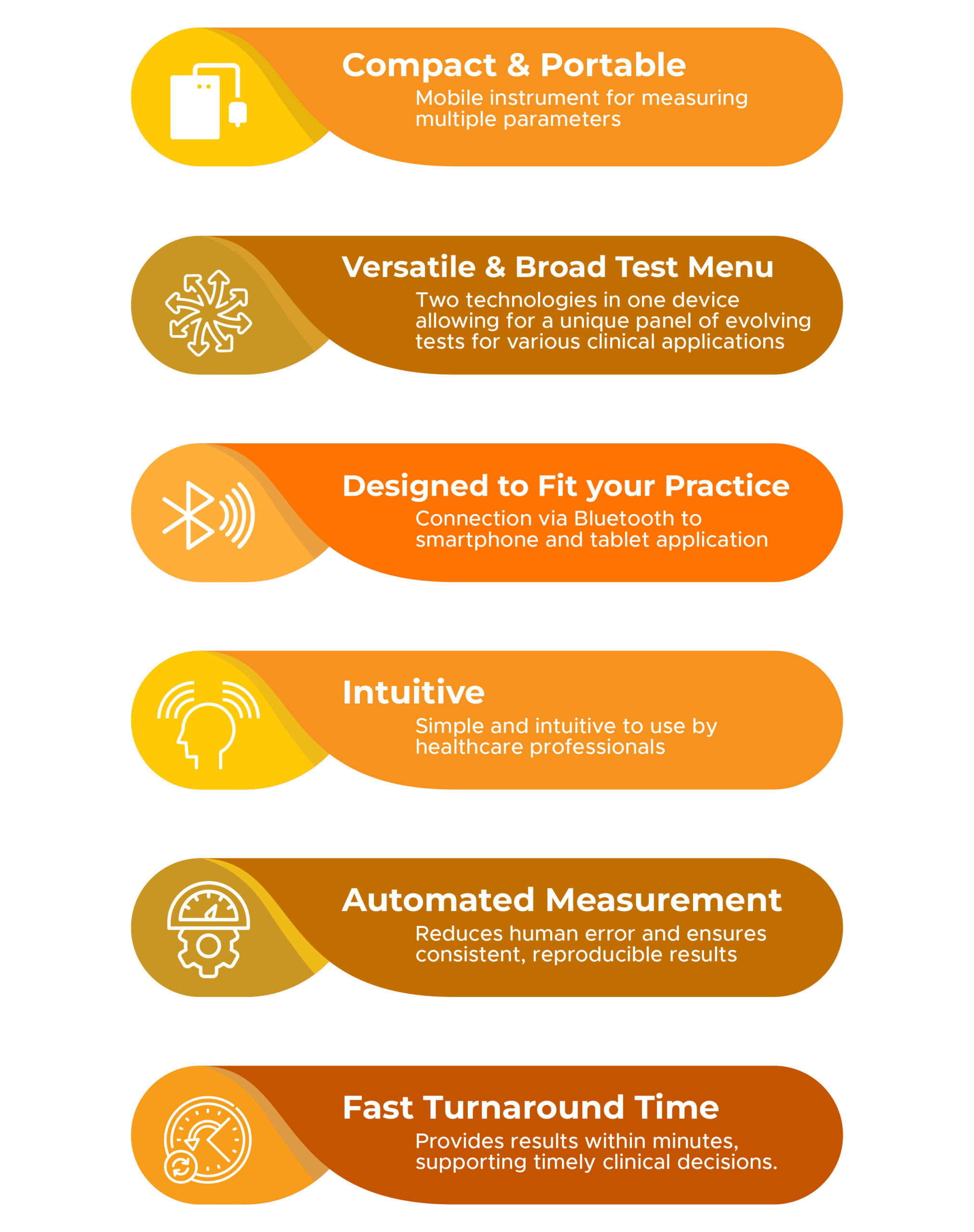
Unique Features of LabPad® Evolution
- Compact & Portable: Easy to transport and set up in diverse healthcare settings
- Versatile & Broad Test Menu: Two technologies in one device allowing for a unique panel of evolving tests for various clinical applications
- Designed to Fit your Practice: Connection via Bluetooth to smartphone and tablet application
- Intuitive: Simple and intuitive to use by healthcare professionals
- Automated Measurement: Reduces human error and ensures consistent, reproducible results.
- Fast Turnaround Time: Provides results within minutes, supporting timely clinical decisions.
Available Test Parameter
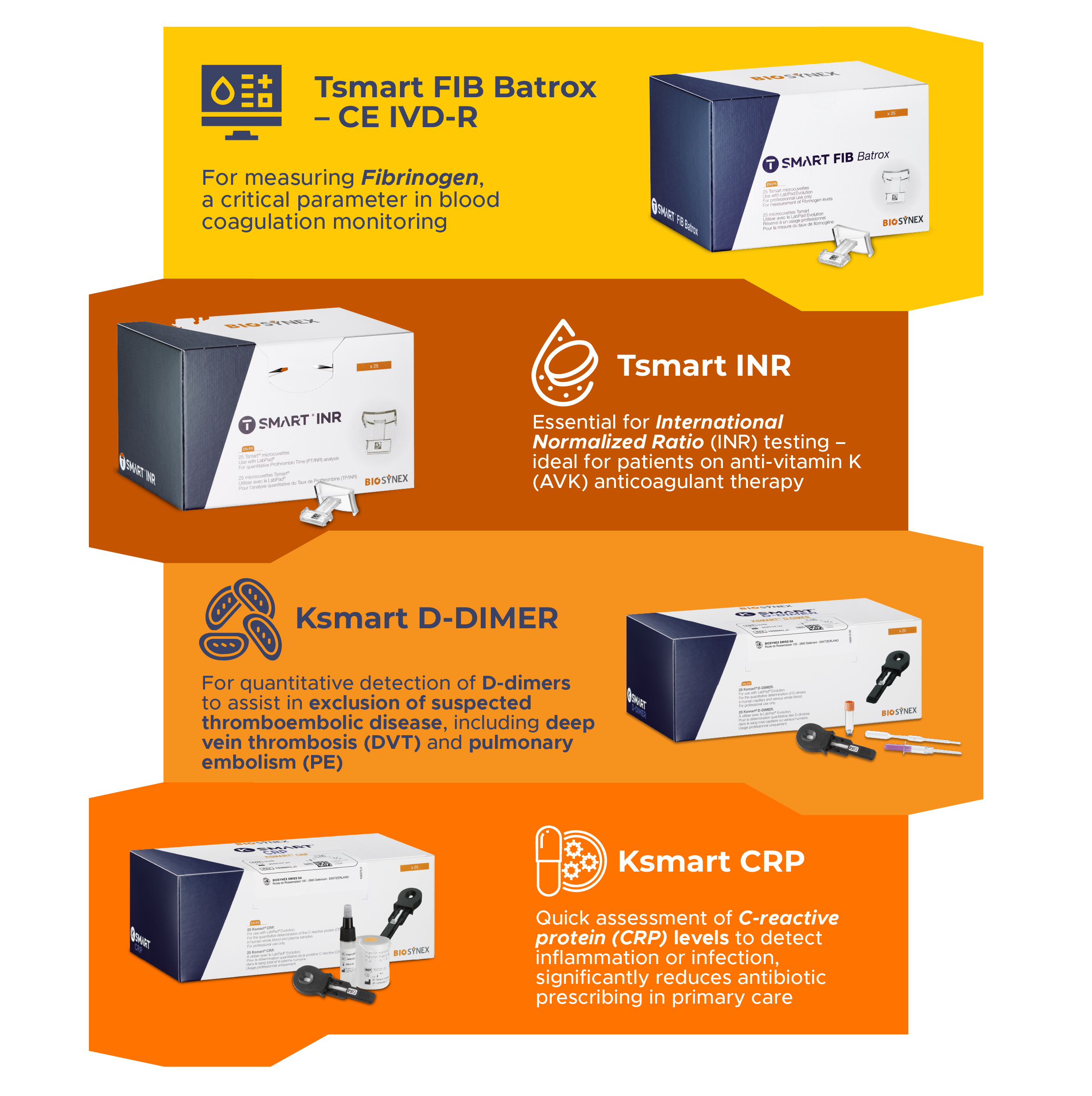
- Tsmart FIB Batrox – CE IVD-R
For measuring Fibrinogen, a critical parameter in blood coagulation monitoring - Tsmart INR
Essential for International Normalized Ratio (INR) testing – ideal for patients on anti-vitamin K (AVK) anticoagulant therapy - Ksmart D-DIMER
For quantitative detection of D-dimers to assist in exclusion of suspected thromboembolic disease, including deep vein thrombosis (DVT) and pulmonary embolism (PE) - Ksmart CRP
Quick assessment of C-reactive protein (CRP)levels to detect inflammation or infection, significantly reduces antibiotic prescribing in primary care
Registered Products in Indonesia
|
Cat. No. |
Product Description |
Intended Use |
Packing Size |
|---|---|---|---|
|
8001661 |
LABPAD Evolution |
Automated measuring test device to be paired with single use compatible tests. |
1 unit |
|
1040012 |
Ksmart CRP |
Quantitative determination of CRP levels |
25T/kit |
|
1040012_C |
Ksmart® CRP + Controls |
Quantitative determination of CRP levels |
25T/kit |
|
1050003 |
Ksmart D-Dimer |
Quantitative detection of D-dimer |
25T/kit |
|
1050003_C |
Ksmart® D-Dimer + Controls |
Quantitative detection of D-dimer |
25T/kit |
|
860617 |
Tsmart FIB Batrox |
Quantitative determination of fibrinogen levels. |
25T/kit |
|
8014825 |
Tsmart INR |
Quantitative determination of Prothrombin Time (PT), INR value (International Normalized Ratio) and Quick Time (QT). |
25T/kit |


