RVR DENGUE RAPID TEST KIT
RVR DENGUE COMBO NS1 & IgG/IgM RAPID TEST
MDA registration no. IVDC7900739717
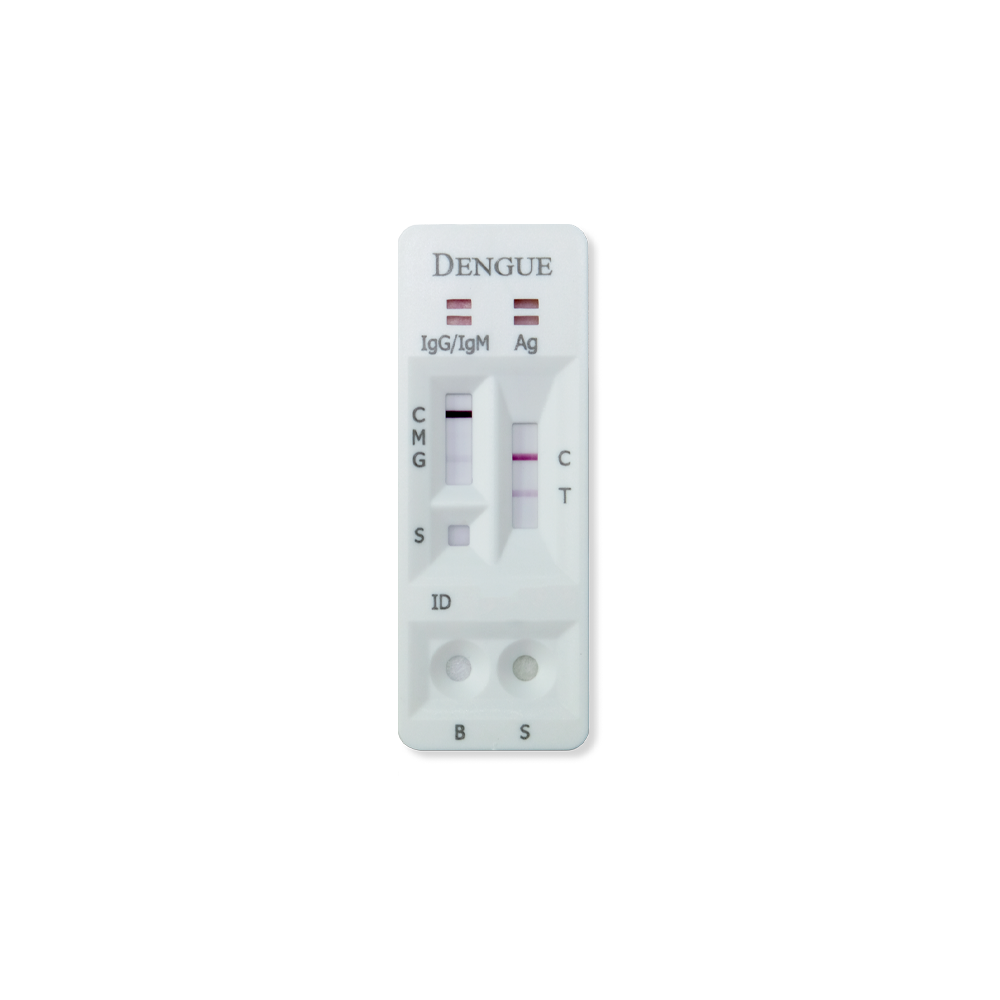
The RVR Dengue Combo NS1 & IgG/IgM Rapid Test is a lateral flow immunoassay for the simultaneous detection and differentiation of IgG anti–dengue virus, IgM anti-dengue virus and dengue antigen (Dengue NS1 antigen) in human serum, plasma or whole blood. It is intended to be used by professionals as a screening test and as an aid in the diagnosis of infection with dengue virus. Any reactive specimen with the Dengue Combo NS1 & IgG/IgM Rapid Test must be confirmed with alternative testing method(s).
Full Spectrum Dengue Detection
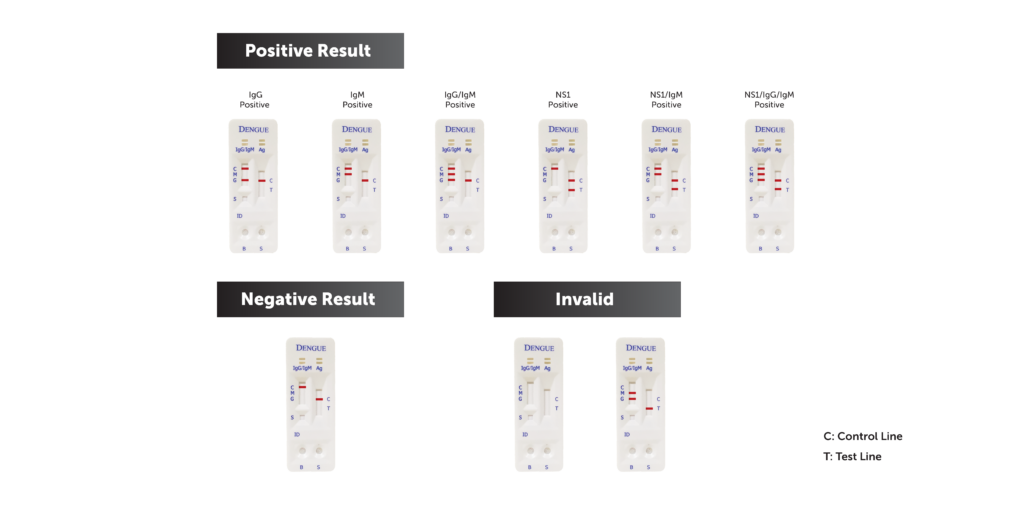
- Early-stage primary infection: NS1 positive
- Late-stage primary infection: IgM and/or IgG positive
- Previous infection: IgG positive
- Acute secondary infection: NS1, IgG, and/or IgM positive
| Specimen | Human whole blood/serum/plasma |
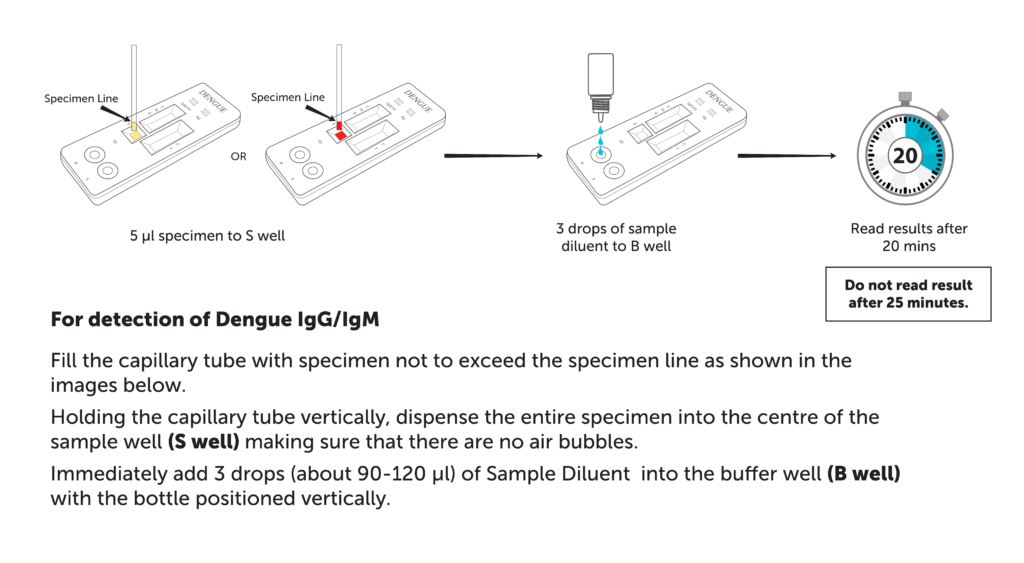
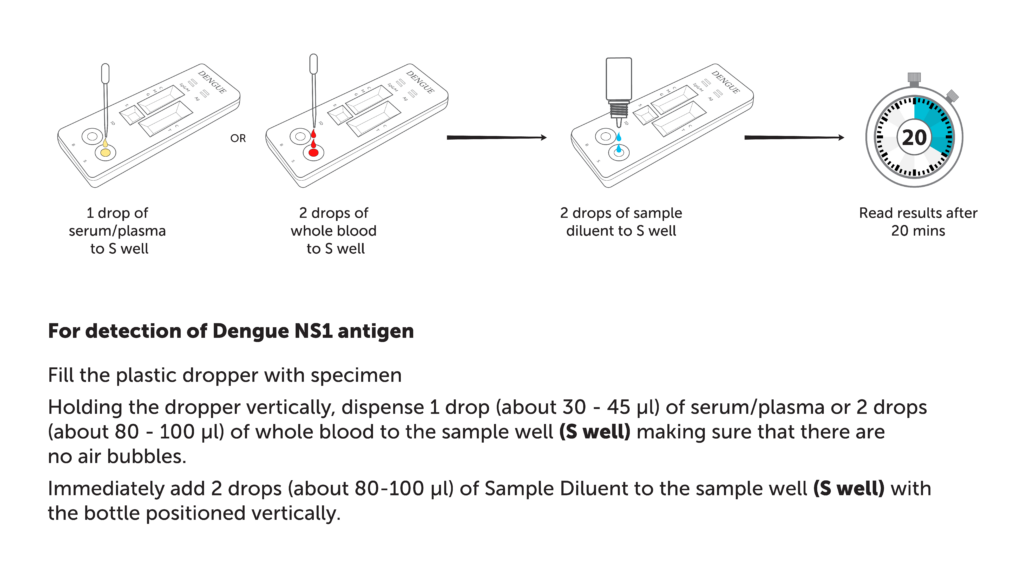
| Specimen volume | For detection of Dengue IgG/IgM
5 µl
For detection of Dengue NS1 antigen 30µl-45 µl (serum/plasma) 80µl-100 µl (whole blood) |
| Specificity | 96.40% |
| Sensitivity | 96.00% |
| Assay time | 20 mins |
|
Cat. No. |
Product Description |
Test Specimen* |
Product Format |
Intended Use |
Intended Use |
Storage Temp. |
Packing Size |
|---|---|---|---|---|---|---|---|
|
RVR011 |
Dengue Combo NS1-IgG/IgM Rapid Test |
WB/S/P |
Cassette |
Detection and differentiation of IgG anti–dengue virus, IgM anti-dengue virus and dengue antigen (Dengue NS1 antigen) |
For Professional Use |
2-30°C |
25 T/ Kit |
* S – Serum; P – Plasma; WB – Whole Blood