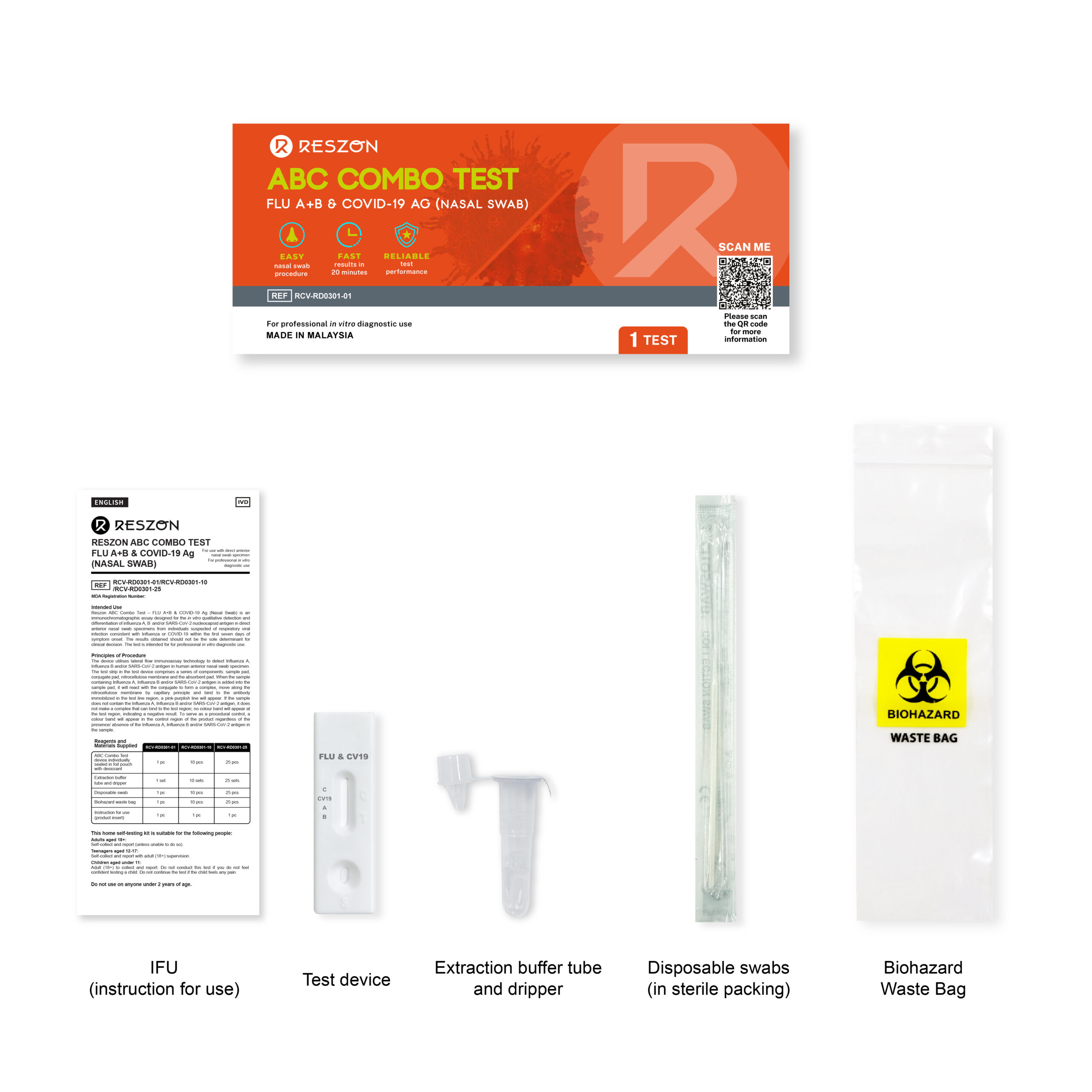
Reszon ABC Combo Test – Flu A+B & COVID-19 Ag (Nasal Swab)
MDA Registration number:
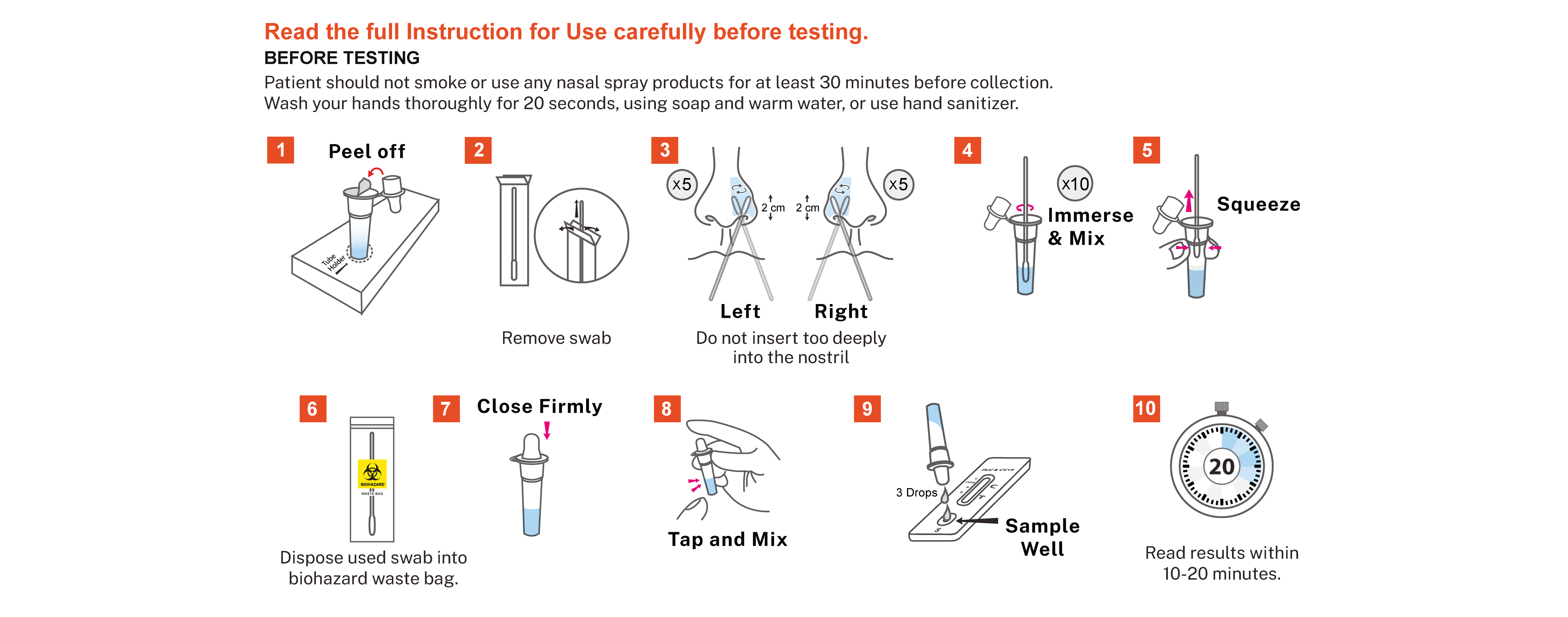
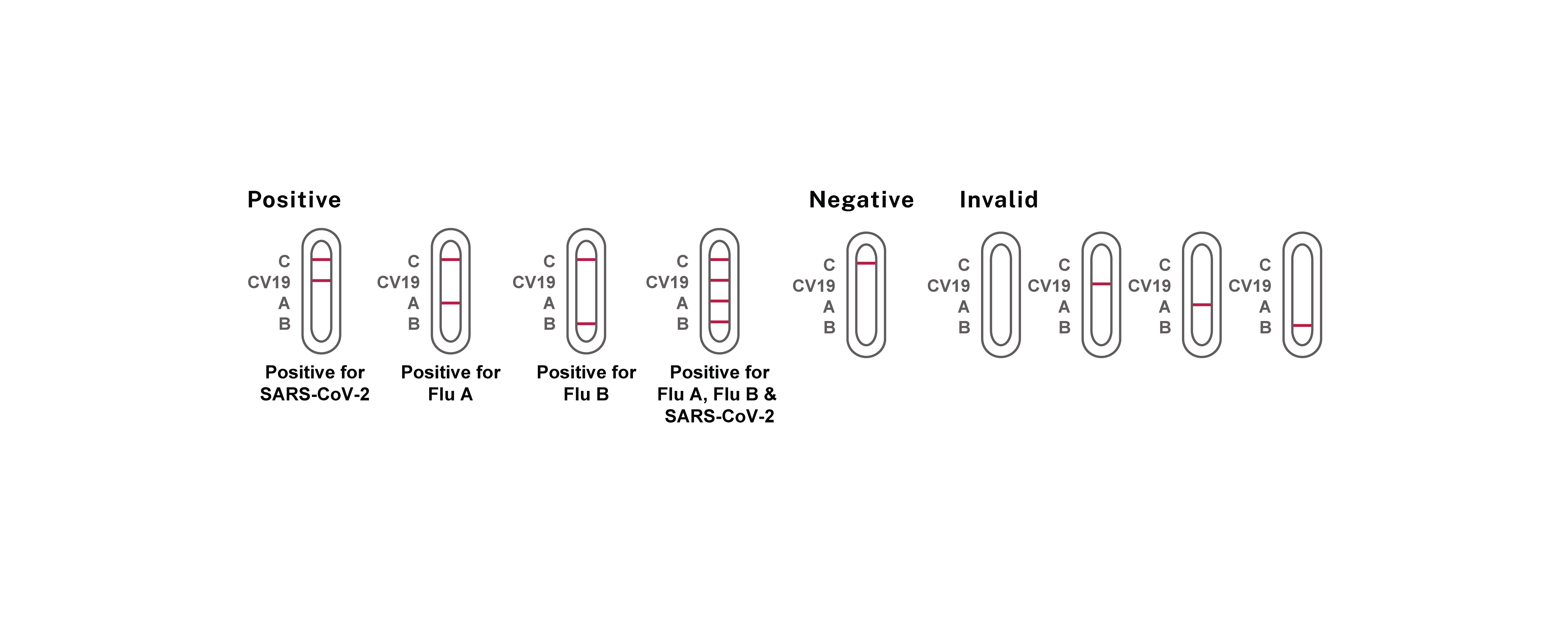
Reszon ABC Combo Test – FLU A+B & COVID-19 Ag (Nasal Swab) is an immunochromatographic assay designed for the in vitro qualitative detection and differentiation of influenza A, B and/or SARS-CoV-2 nucleocapsid antigen in direct anterior nasal swab specimens from individuals suspected of respiratory viral infection consistent with Influenza or COVID-19 within the first seven days of symptom onset. The results obtained should not be the sole determinant for clinical decision. The test is intended for professional in vitro diagnostic use.
| Platform | Immunochromatographic Assay |
| Format | Cassette |
| Detection | Influenza A, B and/or SARS-CoV-2 nucleocapsid antigen |
| Specimen | Direct anterior nasal swab |
| Sensitivity | 91.25% (Influenza A)
92.05% (Influenza B) 96.05% (COVID-19) |
| Specificity | 100% (Influenza A, Influenza B and COVID-19) |
| Assay Time | 20 minutes |
| Shelf Life* | 24 months |
*From date of manufacture
|
Cat. No. |
Product Description |
Test Specimen |
Product Format |
Intended Use |
Intended Use |
Storage Temp. |
Packing Size |
|---|---|---|---|---|---|---|---|
|
RCV-RD00301-01 |
Reszon ABC Combo Test – Flu A+B & COVID-19 Ag |
Nasal swab |
Cassette |
Influenza A, B and/or SARS-CoV-2 nucleocapsid antigen |
For Professional Use |
4-30°C |
1 T/ Kit |
|
RCV-RD00301-10 |
Reszon ABC Combo Test – Flu A+B & COVID-19 Ag |
Nasal swab |
Cassette |
Influenza A, B and/or SARS-CoV-2 nucleocapsid antigen |
For Professional Use |
4-30°C |
10 T/ Kit |
|
RCV-RD00301-25 |
Reszon ABC Combo Test – Flu A+B & COVID-19 Ag |
Nasal swab |
Cassette |
Influenza A, B and/or SARS-CoV-2 nucleocapsid antigen |
For Professional Use |
4-30°C |
25 T/ Kit |