
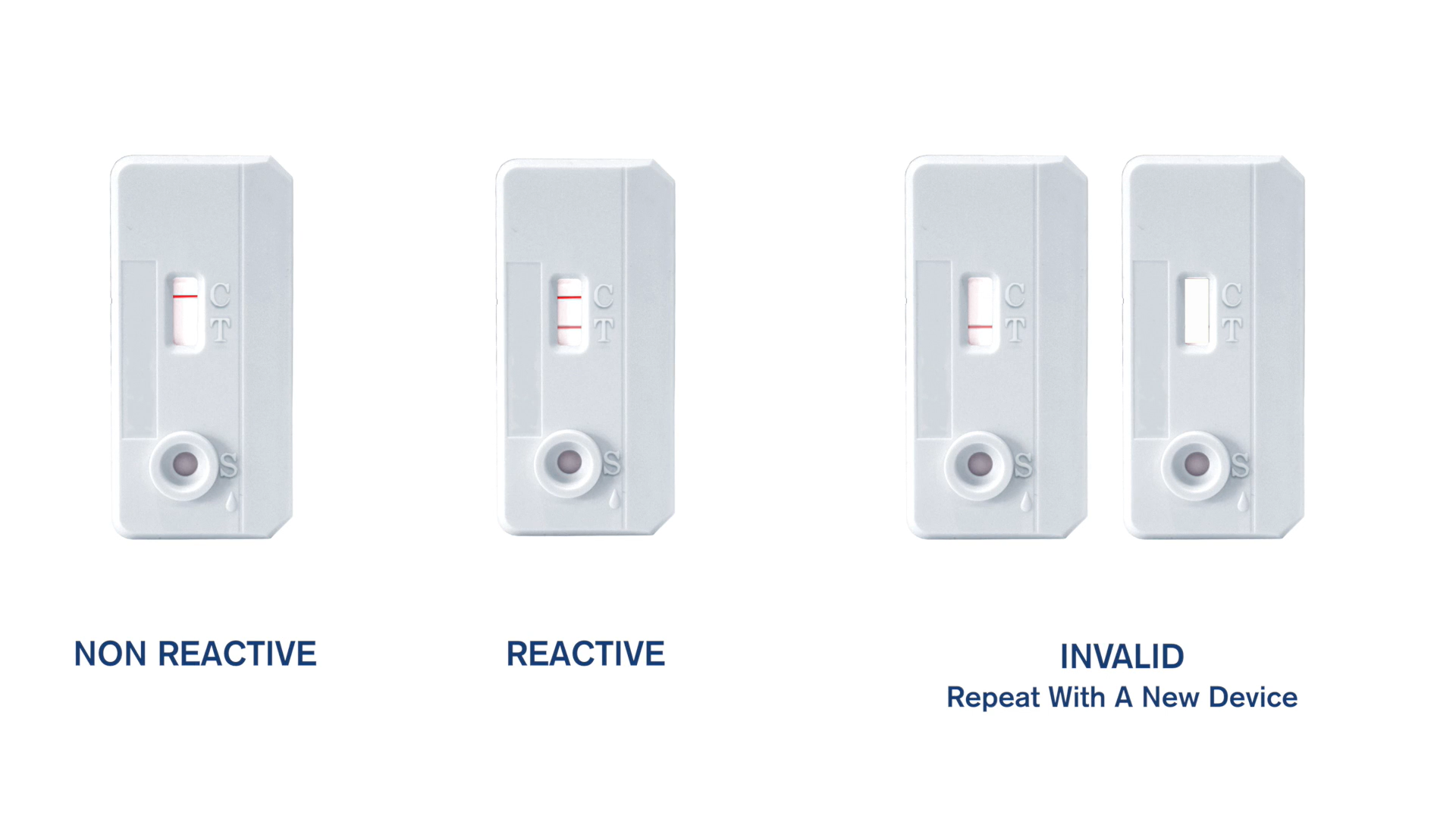
STAT-PAK® HIV 1/2 Assay: Rapid HIV Test in 3 Easy Steps
CHEMBIO HIV 1/2 STAT-PAK® Assay
MDA Registration number: IVDD2866999918
-
Small sample volume:5μl
-
Sensitivity: 100%; Specificity: 100%²
-
Built-in procedural control
1 Kosack CS et al. Journal of the International AIDS Society 2017, 20:21345
2 Refer to product insert for details
A rapid point-of-care assay for the detection of HIV-1 and HIV-2 antibodies in fingerstick whole blood, venous whole blood, serum or plasma.
| Platform | Immunochromatographic Assay |
| Format | Cassette |
| Detection | HIV-1 and HIV-2 antibodies |
| Specimen | Whole blood, serum & plasma |
| Sensitivity | 100% |
| Specificity | 100% |
| Assay Time | 15 minutes |
| Shelf Life* | 24 months |
*From date of manufacture

|
Performance |
Fingerstick |
Venous |
Plasma |
Serum |
|---|---|---|---|---|
|
Sensitivity |
100% |
100% |
100% |
100% |
|
Specificity |
100% |
100% |
100% |
100% |
|
Cat. No. |
Product Description |
Test Specimen |
Product Format |
Intended Use |
Intended Use |
Storage Temp. |
Packing Size |
|---|---|---|---|---|---|---|---|
|
60-9500-0 |
HIV 1/2 STAT-PAK® Assay |
WB/S/P |
Cassette |
Detection of antibodies of HIV Type 1 and 2 |
For Professional Use |
8-30°C |
20 T/ Kit |



