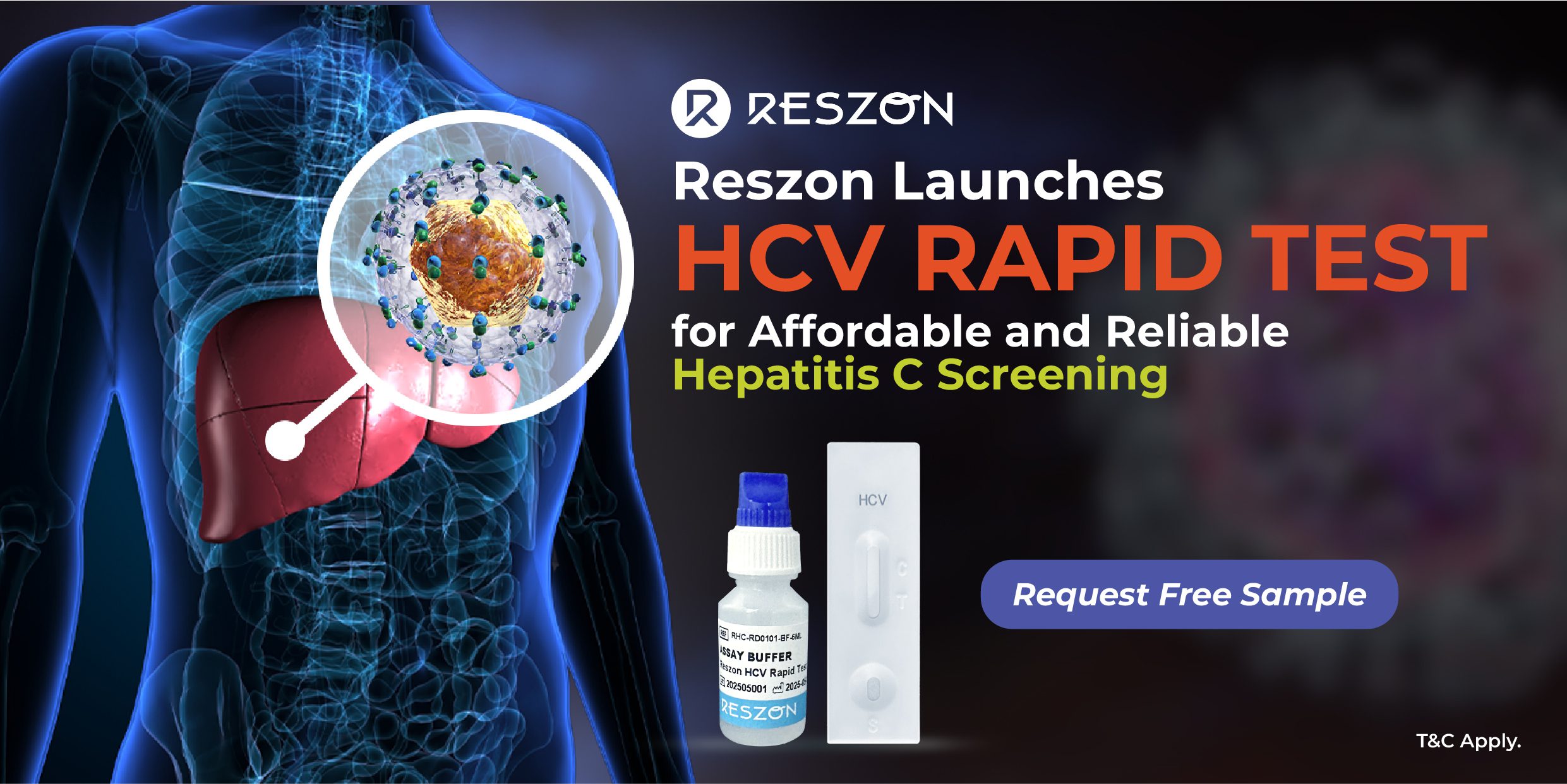
Selangor, 19 Sep 2025 – Reszon Diagnostics International Sdn. Bhd. (“Reszon Diagnostics”), a subsidiary of Hextar Healthcare, manufacturer of innovative rapid diagnostic tests, is pleased to announce the commercial launch of the RESZON HCV Rapid Test (MDA Registration Number: IVDD10681325-211023), designed for professional use in healthcare settings.
The RESZON HCV Rapid Test is a rapid immunochromatographic assay for the qualitative detection of antibodies to Hepatitis C Virus (HCV) in human whole blood, serum, or plasma specimens. It is able to deliver accurate results within 15–20 minutes without the need for special equipment — making it highly suitable for point-of-care testing, public & private health centres, decentralized and resource-limited settings (mobile clinics, outreach programs and rural healthcare facilities).
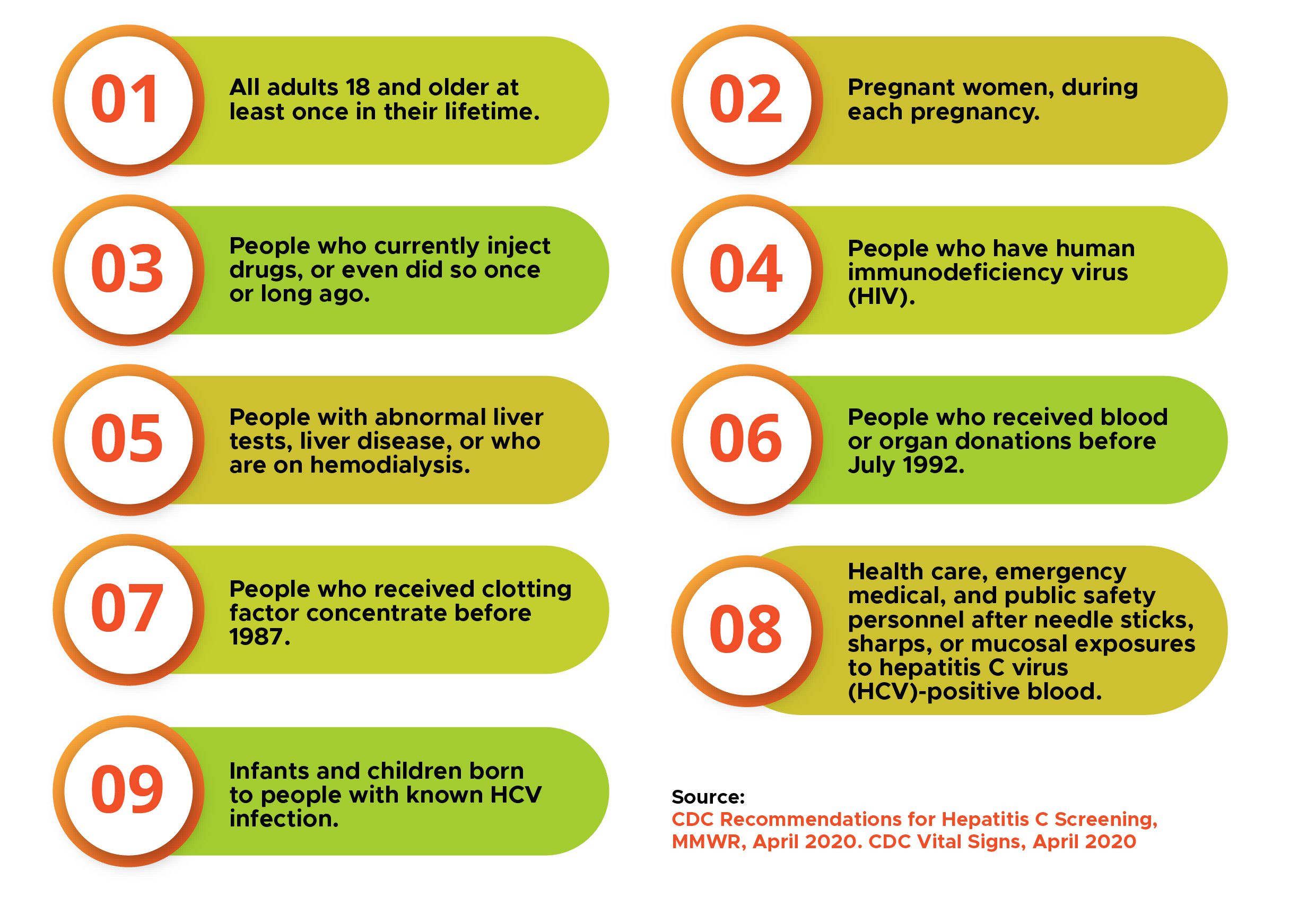
Who Should Get Tested for Hepatitis C?
The Global Burden of Hepatitis C
Hepatitis C remains a major global public health challenge. The World Health Organization (WHO) estimates that 50 million people worldwide are living with chronic HCV infection, with nearly 1 million new infections annually, and approximately 242,000 HCV-related deaths reported in 2022

Source: WHO, 25 Jul 2025
Table 1: Countries that represent two thirds of the global disease burden of hepatitis C, 2022
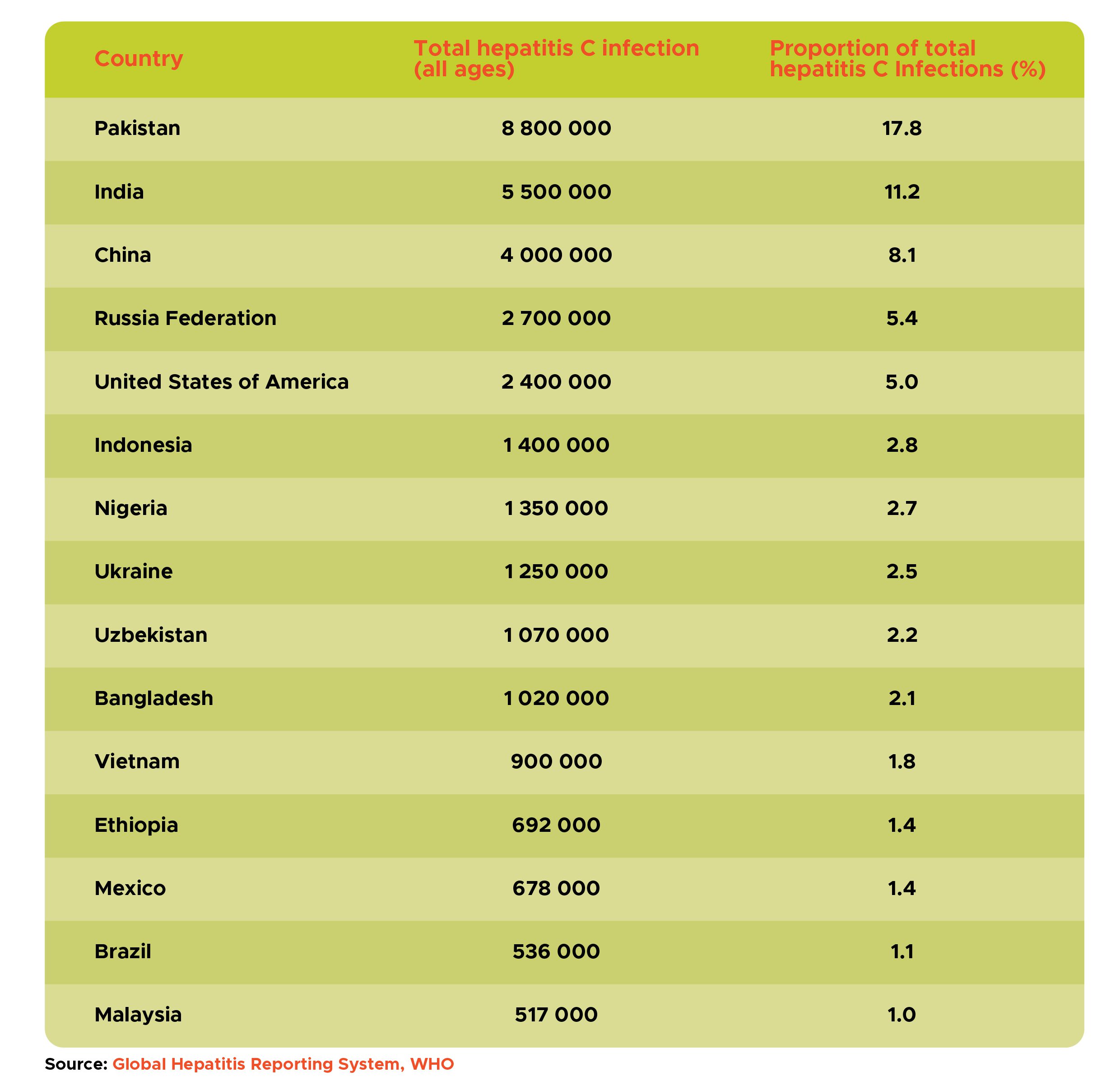
For hepatitis C, six countries – Pakistan, India, China, Russian Federation, United States of America and Indonesia – represent 50% of the global burden; and 15 countries represent about two thirds of the global burden (Table 1).
Despite the availability of curative antiviral therapies, only 36% of people living with hepatitis C had been diagnosed between 2015 and 2022, and 20% had received curative treatment, highlighting the urgent need for expanded access to testing and diagnosis.
Expanding Access to Early Hepatitis C Screening
Early detection and diagnosis play a critical role in reducing disease burden and preventing long-term complications such as liver cirrhosis and hepatocellular carcinoma.

With its ease of use, rapid turnaround time, and reliable performance, the Reszon HCV Rapid Test provides healthcare professionals with a valuable tool for screening and early diagnosis of Hepatitis C, especially in settings where access to laboratory facilities is limited.
“We designed the Reszon HCV Rapid Test to empower healthcare providers with a simple, affordable and accurate screening tool that can be deployed anywhere — from hospitals to clinics and health facilities in rural areas,” said Ms.Katherine Koay, Sales Director of Reszon Diagnostics.
“By bringing rapid testing closer to the community, we help reduce diagnostic gaps, enable early detection, and support global and regional efforts to eliminate Hepatitis C as a public health threat,” said Sales Director, Ms. Katherine Koay.
Want to Learn More About the Reszon HCV Rapid Test?
References
- World Health Organization (2025) Hepatitis C – Fact sheet (updated 25 July 2025). WHO. Available at: https://www.who.int/news-room/fact-sheets/detail/hepatitis-c (Accessed: 19 September 2025).
- World Health Organization (2024) Global hepatitis report 2024: action for access in low- and middle-income countries. WHO. Available at: https://www.who.int/publications/b/68511 (Accessed: 19 September 2025)..
- Centers for Disease Control and Prevention (CDC) (2025) Testing for Hepatitis C. Available at: https://www.cdc.gov/hepatitis-c/testing/index.html (Accessed: 19 September 2025).

