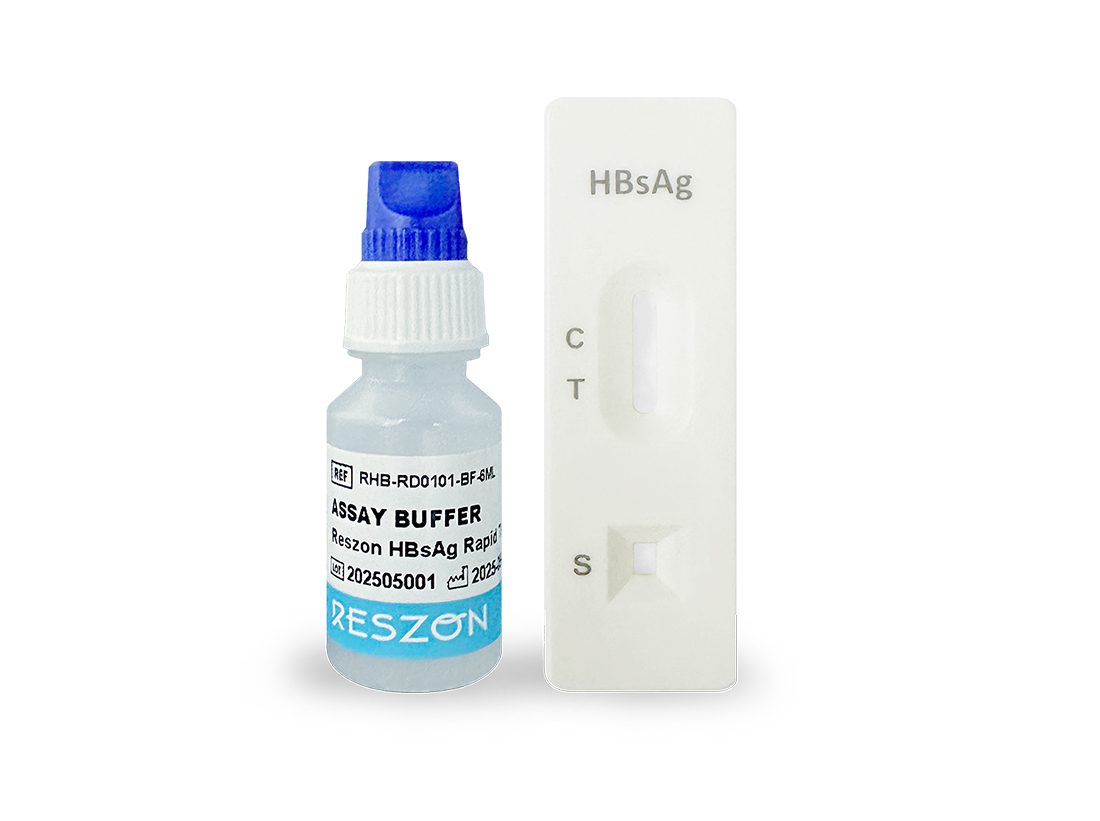
About Reszon Diagnostics
Established in 2010, Reszon Diagnostics International Sdn. Bhd. (“Reszon”) is a Malaysia-based manufacturer and distributor of innovative in vitro diagnostic (IVD) rapid test kits for infectious disease detection, drug of abuse and pregnancy screening, point-of-care testing devices as well as health monitoring devices.
Backed by ISO 13485:2016 quality management system and GDPMD certifications, Reszon is committed to delivering high-quality, accurate, and user-friendly diagnostic solutions for global healthcare markets. Our products are trusted by medical professionals worldwide and are supported by strong in-house R&D as well as strategic collaborations with commercial and academic research partners.
For over a decade, we have honed our expertise in immunochromatographic assay development and innovation, bringing these advancements to the commercial market. Guided by our motto, “Reasoning Possibilities in Life,” we are dedicated to creating new opportunities to enhance human health.
Year of Establishment
Production Area (sq. ft.)
Overseas Market
Product Category
Rapid Testing Solutions for Medical Diagnostics
Discover reliable rapid test kits, point-of-care testing (POCT) device and health motoring device for healthcare providers, point-of-care testing, and self-testing—designed for fast and accurate diagnostics.

Rapid Test Kits for Drug of Abuse (DOA)/ Substance Misuse Screening
Rapidly screen for a wide range of illicit and prescription drugs using urine samples. RESZON multi-drug panels can include adulterant detection and target drug substances —ideal for workplace, clinical, and rehabilitation drug screening programs.

Point-of-Care Testing (POCT) Device
LabPad Evolution from BioSynex (France), the smart portable testing device made for busy clinician and general practitioner (GP) like you. Get accurate lab-quality results in minutes – wherever your patients are.
Available Tests: Fibrinogen (FIB Batrox), INR (for patients on AVK therapy), D-DIMER (rule out DVT & PE) and CRP (inflammation & infection check)
Popular Rapid Test Kits
Discover top-selling rapid tests for COVID-19, influenza, dengue, HIV, drugs of abuse, typhoid, filariasis, and pregnancy detection
Latest Products
Discover the latest innovations and product offerings from Reszon, featuring new rapid test kits, POCT devices, and health monitoring solutions
Product Video
Watch quick video tutorial guide to learn how to use our products
Browse all videos on our Youtube channel
Latest News & Events
Would you like to know more? Then get in touch now!