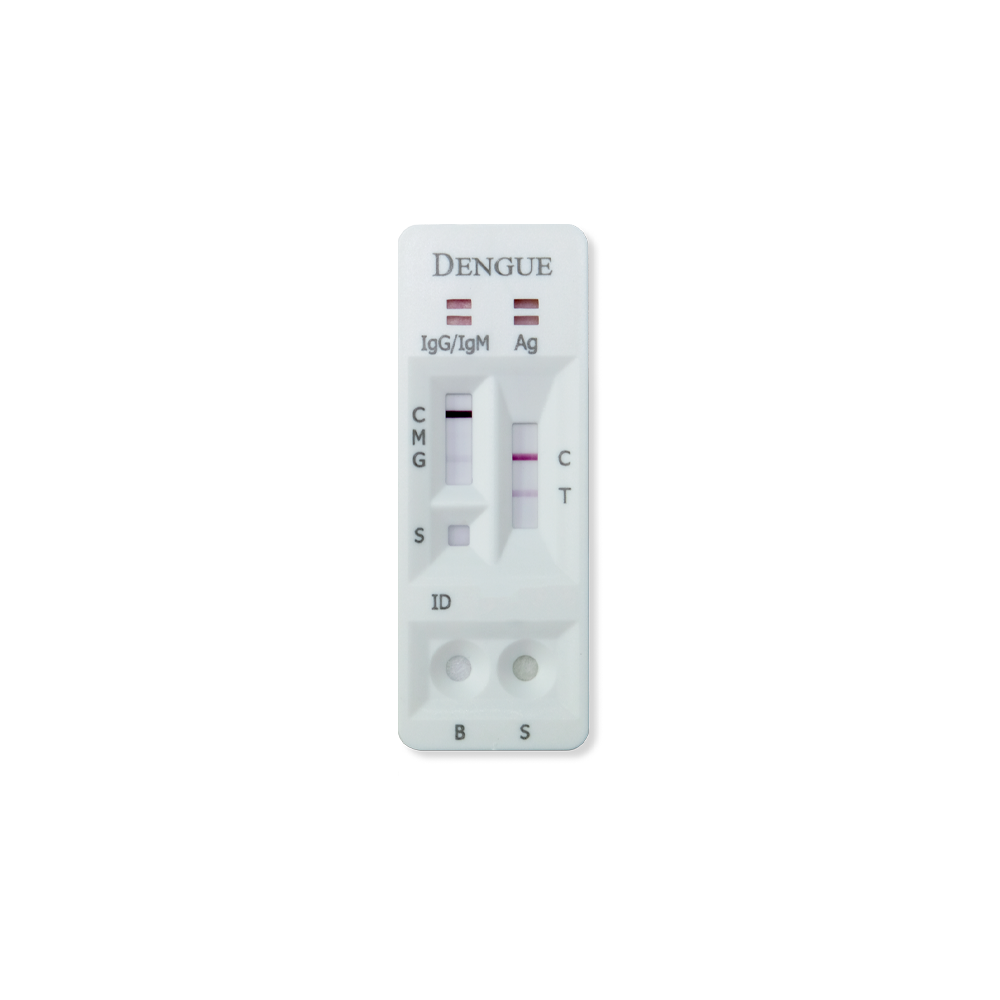
RVR DENGUE RAPID TEST KIT
RVR DENGUE COMBO NS1 & IgG/IgM RAPID TEST
MDA registration no. IVDC7900739717
The RVR Dengue Combo NS1 & IgG/IgM Rapid Test is a lateral flow immunoassay for the simultaneous detection and differentiation of IgG anti–dengue virus, IgM anti-dengue virus and dengue antigen (Dengue NS1 antigen) in human serum, plasma or whole blood. It is intended to be used by professionals as a screening test and as an aid in the diagnosis of infection with dengue virus. Any reactive specimen with the Dengue Combo NS1 & IgG/IgM Rapid Test must be confirmed with alternative testing method(s).
Full Spectrum Dengue Detection
- Early-stage primary infection: NS1 positive
- Late-stage primary infection: IgM and/or IgG positive
- Previous infection: IgG positive
- Acute secondary infection: NS1, IgG, and/or IgM positive
| Specimen | Human whole blood/serum/plasma |
| Specimen volume | For detection of Dengue IgG/IgM
5 µl For detection of Dengue NS1 antigen 30µl-45 µl (serum/plasma) 80µl-100 µl (whole blood) |
| Specificity | 96.40% |
| Sensitivity | 96.00% |
| Assay time | 20 mins |

|
Cat. No. |
Product Description |
Test Specimen* |
Product Format |
Intended Use |
Intended Use |
Storage Temp. |
Packing Size |
|---|---|---|---|---|---|---|---|
|
RVR011 |
Dengue Combo NS1-IgG/IgM Rapid Test |
WB/S/P |
Cassette |
Detection and differentiation of IgG anti–dengue virus, IgM anti-dengue virus and dengue antigen (Dengue NS1 antigen) |
For Professional Use |
2-30°C |
25 T/ Kit |
* S – Serum; P – Plasma; WB – Whole Blood
Frequently Ask Questions (FAQ) for RVR Dengue Combo NS1 & IgG/IgM Rapid Test (Serum/Plasma/Whole Blood)
Before running the test
1. Can I use the test device if the pouch is damaged or there is broken/ incomplete seal?
No. Do not use the test device if the pouch is damaged or the seal is broken/ incomplete. Please use a new test.
2. Can I use the test device if there is no desiccant in the pouch?
No. Desiccant should come together with test device in the pouch. Please use a new test.
3. Can I use the kit beyond its expiry date?
No. User should not use the test beyond the expiry date shown on the test kit labelling.
While running the test
4. Can I use specimen directly from the refrigerator?
No. Refrigerated specimen should be equilibrated to room temperature (about 30 minutes) or between 15°C and 30°C before being tested. Remember to mix the specimen before use.
5. How to mix the specimen?
Mix the specimen by gently inverting the tube 5-10 times. DO NOT vortex the specimen. Specimens containing visible particulate matter should be clarified by centrifugation before testing.
6. Can I use haemolyzed specimen?
No. Haemolyzed specimen cannot be used on this test. Haemolyzed samples may interfere with test results.
7. Can I use whole blood sample from finger prick?
Yes. Whole blood sample from finger prick can be used. However, skilful professional user and a suitable lancet are required to ensure sufficient specimen can be collected. It is highly recommended to use whole blood sample from venipuncture.
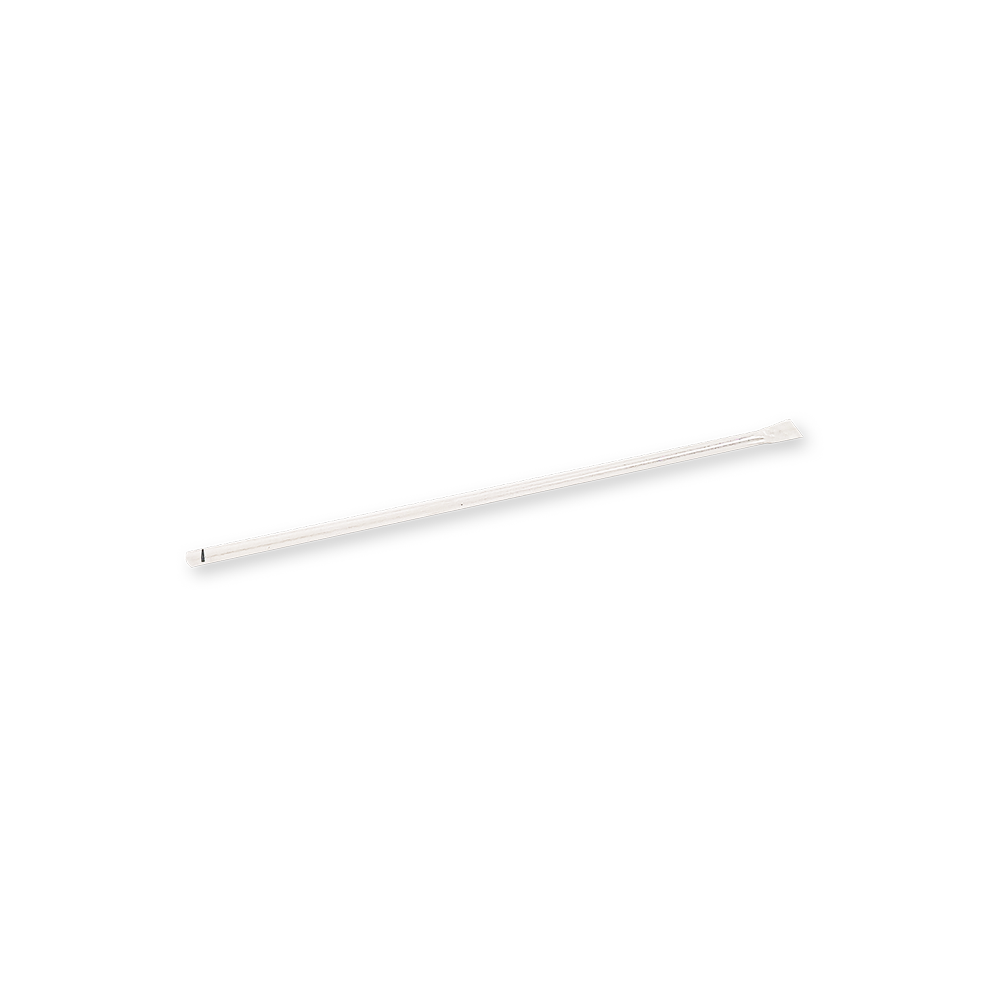
8. How to use capillary tube provided in the kit for the Dengue IgG/IgM test?
Pinching the top with fingers and position the capillary tube into the specimen. Subsequently, lift the finger from the capillary tube slowly and fill the capillary tube with specimen. Make sure not to exceed the specimen line marked on the capillary tube. Then, hold the capillary tube vertically and pinch the top of capillary tube to dispense the entire specimen into the sample well (marked “S”).
For video demo, please visit https://www.youtube.com/watch?v=kocxQLfMP8g
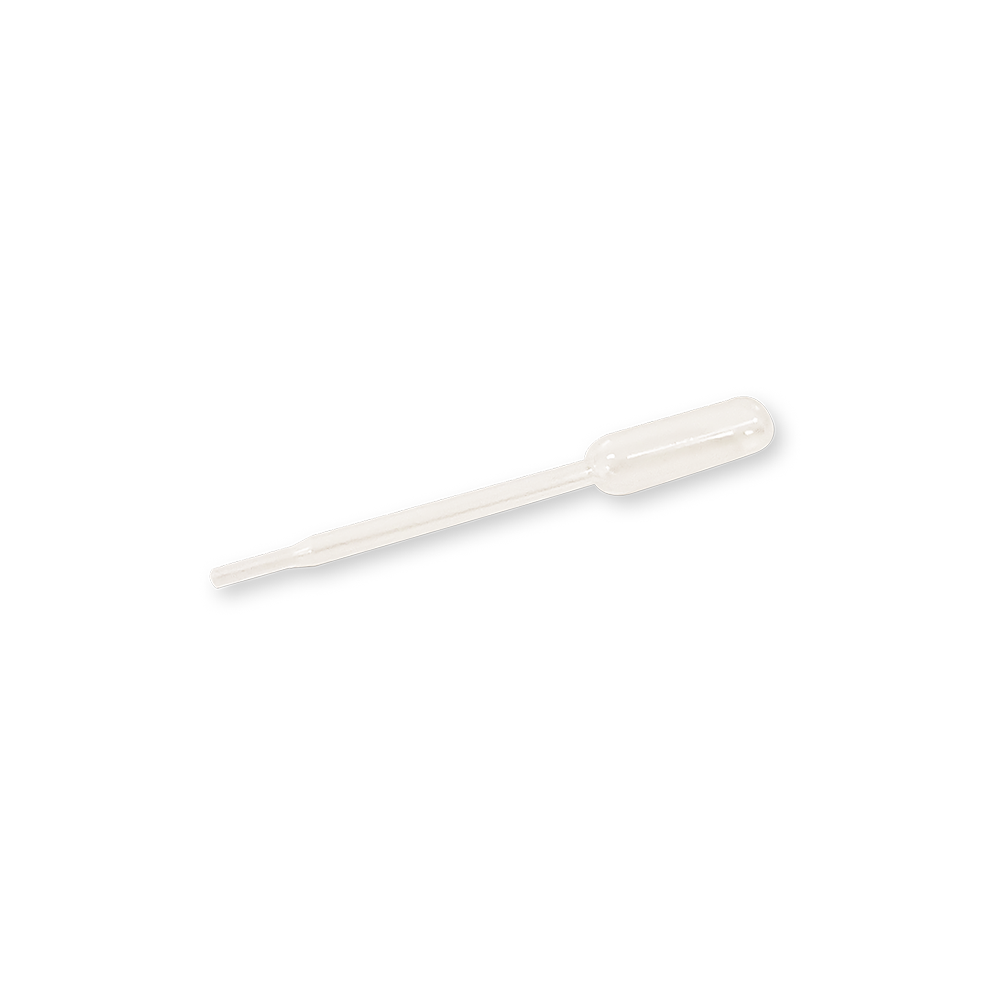
9. How to use plastic dropper provided in the kit for the Dengue NS1 Ag test?
Pinching the bulb at the top with fingers and position the dropper into the specimen. Subsequently, lift the fingers slowly from the bulb to fill the dropper with the specimen. Then, hold the dropper vertically and pinch the bulb of the dropper to dispense 1 drop of serum/plasma, or 2 drops of whole blood (drop by drop) into the sample well (marked “S”). Make sure there are no air bubbles.
For video demo, please visit https://www.youtube.com/watch?v=kocxQLfMP8g
10. Can I use the same capillary tube/ dropper for both Dengue IgG/IgM and NS1 Ag tests?
No. Sample volume needed for Dengue IgG/IgM and NS1 Ag tests are different. User should use the capillary tube provided for Dengue IgG/IgM test and dropper for Dengue NS1 Ag test, as stated in the IFU.
11. Two bottles of buffer are supplied in each test kit. Can I use the same buffer for both tests?
Yes. The 2 buffer bottles provided in the kit are identical. Both Dengue IgG/IgM and NS1 Ag tests are using the same buffer. You may use either one of the buffers in the kit.
12. I found that the buffer is not enough to run all the tests in the kit. Can I use buffer from other brand or other test kit?
No. Buffer from other brand or other test kit cannot be used as a substitute for the buffer in this kit. Please contact your supplier for assistance. The buffers provided in the kit are more than the actual volume needed to complete all 25 tests in a kit. User should check the IFU again and ensure that the tests are run following the correct assay procedure.
13. Can I put more sample to the test? How if I accidentally added more sample to the test?
No. User should follow the instruction of the assay procedure strictly. Failure to follow the instruction may give inaccurate test results. Repeat the test if the sample volume is incorrect during the testing.
14. How if I accidently put the specimen at the well “B” or buffer at well “S” for Dengue IgG/IgM test?
Specimen must be added to well “S” while buffer at well “B”. If you did it wrongly, discard the test and repeat with a new test device. The tests should be run as per instructions stated in the IFU.
15. Why is it the sample never flow up, or the sample only flow half of the test window?
It may be due to insufficient specimen or buffer volume. Sufficient amount of specimen and buffer are important to ensure the sample to flow up properly. Please repeat the test with a new test device if invalid results observed.
User should read the assay procedure in the IFU provided carefully before running the test. User should ensure that the correct volume of specimen is used during the testing.
- If using a micropipette, make sure 5 μl of specimen for IgG/IgM test, 60 μl of serum/plasma or 70 μl of whole blood for NS1 test are used.
- If using capillary tube for IgG/IgM test, make sure sample taken until the specimen line and dispense entire specimen into the well “S”
- If using dropper for NS1 Ag test, make sure 2 full drops of serum/plasma or 2 full drops of whole blood is dispensed into the well “S”
- For whole blood specimen, the sample pad area at well “S” will be fully covered with blood sample if the correct amount of specimen is added
When adding buffer, it is important to hold the buffer bottle vertically, carefully press the bottle to release full drop of buffer, one drop by one drop. The droplet volume of the buffer may be inconsistent if the buffer bottle is not hold vertically above the well “S”, or buffer being release too fast.
In rare cases, sample flow problem may also occur if too much specimen or buffer were added into well “S” or well “B”. Excessive sample may block the well and inhibit the sample flow to the sample pad.
16. Can we add more buffer if the sample is not flowing up?
No. The assay procedures need to be followed strictly. If no sample flow observed, check again the procedure and repeat the test with a new test device
17. Can I read results before 20 minutes?
Only positive results can be read before 20 minutes. Positive results that appear before 20 minutes can be considered as positive. Negative result must be confirmed at 20 minutes.
18. Can I read results after 30 minutes?
No. Do not read results after 25 minutes. Results read after 25 minutes may lead to a false positive, false negative, or invalid result.
Interpretation of results
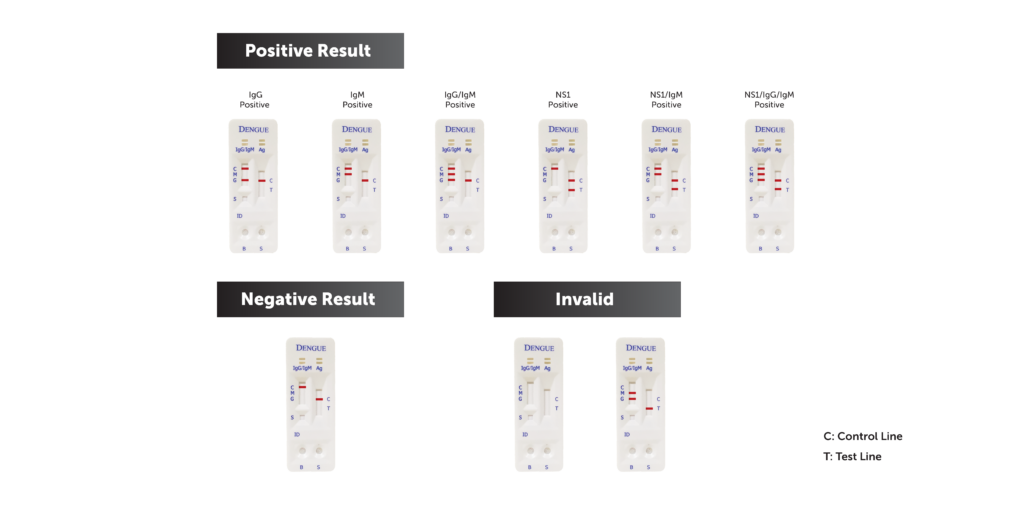
19. What do the results mean?
If coloured bands appear at the control line (C) and test lines (T, IgG, and/or IgM), it indicates a positive result. Positive results indicate the presence of Dengue NS1 antigen and Dengue antibodies in the specimen. Positive result should not be used as the sole criteria for the diagnosis of Dengue. Positives results should be confirmed by alternative testing method and clinical finding before positive determination is made.
If only control line (C) is visible, no coloured line developed at positions T, IgG or IgM. No IgG or IgM antibodies or NS1 antigen were detected. Negative result does not preclude the possibility of exposure to or infection with dengue viruses. Negative result can occur if the quantity of dengue NS1 antigen or antibody present in specimen is below the detection limit of the test, or the dengue antigen that are detected are not present during the stage of disease in which sample is collected.
|
NS1 antigen |
IgM antibody |
IgG antibody |
Suggestive of: |
|---|---|---|---|
|
Positive |
Negative |
Negative |
Acute phase/ early infection |
|
Positive |
Positive |
Negative |
Primary/ Acute phase infection |
|
Negative |
Positive |
Negative |
Primary initial stage infection/ early recovery phase |
|
Positive |
Positive |
Positive |
Acute/ recurrent infection |
|
Negative |
Positive |
Positive |
Recent/ recurrent infection |
|
Negative |
Negative |
Positive |
Past infection |
|
Positive |
Positive/ Negative |
Positive |
Secondary infection – Acute phase |
|
Negative |
Negative |
Negative |
Not dengue infection, further investigation to be done |
20. What to do if the results showed negative result, but the symptoms persist?
If the symptom persists, while the result is negative or non-reactive result, it is recommended to re-sample the patient few days later or test with an alternative testing method such as PCR, ELISA.
After running the test
21. Can I store my kit in refrigerator?
Yes. The test kit can be stored at a temperature between 2-30 °C. Do not freeze the kit or its components and do not store the kit under direct sunlight.
When stored in a refrigerator, all kit components must be equilibrated to room temperature (15-30 °C) or a minimum of 30 minutes prior to performing the test.
Good Laboratory Practice
22. How is the good quality control for RVR Dengue Combo Rapid Test?
Users should follow respective Good Laboratory Practice SOP of the labs. However, it is recommended to use external controls, positive and negative, to confirm the test procedure and to verify proper test performance, particularly under the following circumstances:
- New operator uses the kit, prior to performing testing of specimens.
- A new lot of test kits is used.
- A new shipment of test kits is used.
- The temperature used during storage of the kit falls outside of 2-30°C.
- The temperature of the test area falls outside of 15-30°C.
- To verify a higher-than-expected frequency of positive or negative results.
- To investigate the cause of repeated invalid results.